Hallmarks of Biological Failure
Category: Application Area Application Area: Biological Failure
Date/Time: April 8, 2019 - April 10, 2019
Group photo
 (x)
(x)
Organizers
Michael Hochberg (Univ. Montpellier/SFI)
Daniel Promislow (Univ. Washington)
Meeting Highlight
Rozalyn Anderson (Univ. Wisconsin)
Bernie Crespi (Simon Fraser Univ.)
James DeGregori (CU Denver)
Kelley Harris (Univ. Washington)
Morgan Levine (Yale Univ.)
Barbara Natterson-Horowitz (Harvard)
Maria Riolo (SFI)
Ophelie Ronce (UBC)
Marten Scheffer (Wageningen Univ.)
David Schneider (Stanford)
Sabrina Spencer (CU Boulder)
Shripad Tuljapurkar (Stanford)
Dario Riccardo Valenzano (Max Planck)
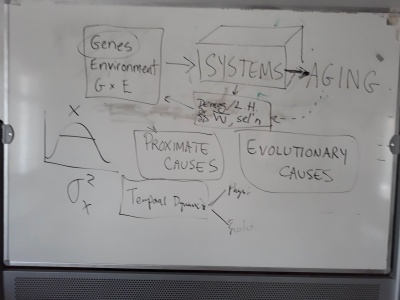
Biological systems eventually fail. Depending on the system type, birth to failure can occur in anywhere from fractions of a second to millennia. All such systems come into existence following often characteristic phases of development, and are either intrinsically resilient to change or are maintained in a high-functioning state via specific mechanisms. Nevertheless, despite what may be stable function over much or most of a system’s lifespan, all systems eventually age, and aging is prognostic for eventual failure. A central question is whether all robust biological systems fail in the same way or in a limited number of ways. A number of approaches have been taken to address this question, but most ignore how hierarchical interdependence in the nodes forming the system network are actually structured, and how this predicts the aging in each node and eventual cascades that lead to modular failure or failure of the whole system.
The working group “Hallmarks of Biological Failure” will bring together experts working on biological systems ranging from subcellular processes to ecosystems to discover if there is a common pattern in the events leading up to system failure, and what is (are) the higher-level driver(s) that restrict the pattern(s) we observe. Our aim is to produce a framework for describing and understanding the precursors (such as aging) of biological failure, which integrates the effects of within-generation demographic selection and longer-term natural selection. The results will have implications for systems biology, aging research, gerontology, and evolutionary biology.
Bernie Crespi (Simon Fraser Univ.) - Complexity, Breaking Bad Tradeoffs, and the Evolution of Biological Failure[edit source]
I will discuss two points. First, how are genomic and organismal complexity related to slow, and fast, failure? Do biological systems fail more under conditions of high
complexity and tight coupling, as posited for inanimate systems? Do increases in genomic and organismal complexity result in short-term benefits, but more longer-term evolutionary vulnerabilities? Second, how do tradeoffs mediate failure? Most tradeoffs are 'bad' in that system-wide organismal lifetime optimization is not achieved, even if they are relatively 'good' for propagating genes. Can such bad tradeoffs be broken, artificially, by humans?
I think so, in some cases. I discuss examples, from mental disorders, life histories, and senescence.
Barbara Natterson-Horowitz (Harvard) - Dynamic Cardiovascular Systems, Evolved Adaptations and Clinical Pathology[edit source]
Several forms of high impact human cardiovascular(CV) pathology are related to autonomic dysregulation, emerging in association with adrenergic events. A phylogenetic survey of organisms with spontaneous occurrence of these pathologies and correlation with life history points to the adaptive value of the phenotypic flexibility facilitated by these dynamic systems. It also suggests that varied intra-individual CV physiologic responses to environmental threat are complex, adaptive and play a central role in vulnerability to several forms of human cardiovascular pathology.
James DeGregori (CU Denver) - Explain this! - Evolutionary approaches to unanswered questions in cancer biology[edit source]
I plan to throw out some observations (e.g. age-dependent cancer incidence for different organs and different species), and how these are currently enigmatic. I’ll discuss possible explanations, but also highlight were explanations are currently lacking.
David Schneider (Stanford) - Measuring the resilience of hosts to infections by mapping disease space[edit source]
My group has been trying to find relatively simple multidimensional ways of measuring the response to infections. Our idea is to measure how far a host will be pushed from its normal physiology when it sickens and what route it will take coming back from sickness. We do this by drawing the trajectory infected individuals take through phase space and try to produce maps that improve our understanding of the process. We want to understand how far the system can be pushed before it breaks, which is one sort of system failure. We then want to understand how this varies. For example, do hosts die because their physiology becomes more elastic? In this case they would be more likely to enter physiological states that are not survivable. Alternatively, physiological states that would be survivable when to one host might not be survivable to another. Our first project is to understand what variation looks like when we examine infections this way. As we proceed we would like to model this system more carefully.
Rozalyn Anderson (Univ. Wisconsin) - Metabolic Integrity & Aging: Amplification of Small Perturbations[edit source]
Caloric restriction (CR) delays aging and the onset of age-related disease in diverse species, including nonhuman primates. Emerging data has focused our studies on links between metabolic status and disease vulnerability; several diseases of aging including diabetes, cancer, and neurodegeneration, have an established metabolic component. Candidate factors involved in longevity regulation are nutrient sensitive and interconnected in terms of signaling pathways and downstream effector actions. Molecular profiling of the transcriptome, proteome, and metabolome identifies CR responsive elements that are highly enriched for metabolic pathways. Here too connectivity among responsive nodes, or mega clusters, is complex. Our recent work shows that small changes in metabolic status precipitate large-scale multi-modal functional changes across diverse cellular processes. We suggest that modest failures in metabolic integrity are amplified by such mechanisms with age to broadly impact homeostasis and adaptation, creating shared vulnerability to diseases and conditions despite differences in their etiology.
Shripad Tuljapurkar (Stanford) - Models in Aging: Two Examples[edit source]
I discuss approaches to two problems on very different timescales. For a single lifetime, transitions between states of health (disability) can be viewed as stochastic movement out of a potential with two minima. Aging can mean changes in the amplitude of noise, depth of potential, or width of potential. Such dynamics are conceptually similar to the disability transition in current medical understanding. What are the math features? Can we make this into a statistical model? On evolutionary timescales, post-reproductive life can evolve according to varipus arguments that are all examples of “borrowed fitness.” I explain what this means and mainly ask what questions we should be asking.
Marten Scheffer (Wageningen Univ.) - Quantifying Resilience of Humans and other Animals[edit source]
All life requires the capacity to recover from challenges that are as inevitable as they are unpredictable. Understanding this resilience is essential for managing the health of humans and their livestock. It has long been difficult to quantify resilience directly, forcing practitioners to rely on indirect static indicators of health. However, measurements from wearable electronics and other sources now allow us to analyze the dynamics of physiology and behavior with unsurpassed resolution. The resulting flood of data coincides with the emergence of novel analytical tools for estimating resilience from the pattern of micro-recoveries observed in natural time series. Such dynamic indicators of resilience (DIORs) may be used to monitor the risk of systemic failure across systems ranging from organs to entire organisms. These tools invite a fundamental rethink of our approach to the adaptive management of health and resilience.
Dario Riccardo Valenzano (Max Planck) - Relaxed selection shapes the rate of aging across species[edit source]
African killifishes independently evolved annual life cycles at least three times, offering a unique natural experiment of diversification of life history strategies. Using a comprehensive whole-genome sampling of 46 species of African killifishes, we found that genome size correlates with annual life style and climate. Annual species had genome-wide expansion of transposable elements, higher gene family turn-over rates and relaxed selection in genes in known aging pathways, such as mitochondrial replication and translation, mTOR pathway and DNA repair. Whole-genome resequencing in wild Nothobranchius populations showed bottle-necks and a genome-wide signature of relaxation of selection in populations evolved in dryer climates. In conclusion, evolution in ephemeral environments in African killifishes caused an extensive relaxation of selective constraints at genome-wide level. We discovered that, in African killifishes, ecology drove the evolution of short life span and rapid aging, associated to tens of thousands of slightly deleterious mutations driven to high frequencies.
Sabrina Spencer (CU Boulder) - Single-cell analysis of heterogeneity in proliferation-quiescence decisions[edit source]
Research in the Spencer lab is focused on understanding how signaling events control cell fate. Studying these processes in single cells reveals remarkable cell-to-cell variability in response to stimuli, even among genetically identical cells in a uniform environment. We seek to understand the sources and consequences of this heterogeneity in the cellular response to stimuli. The stimuli we study include growth factors, cell stress, and targeted cancer therapeutics. To do this, we develop genetically encoded fluorescent sensors for signaling events of interest. We then use long-term live-cell microscopy and cell tracking to quantify the dynamics of upstream signals and link them to cell fate (proliferation, quiescence, apoptosis, senescence). Our long-term goal is to understand the normal mechanistic functioning of signaling pathways that control proliferation, to understand how these signals go awry in cancer, and eventually to alter the fate of individual cells.
Morgan Levine (Yale Univ.) - Systems-Level Modeling of Aging across Biological Levels of Organization[edit source]
Aging is associated with numerous changes at all levels of biological organization. Harnessing this information to develop measures that accurately and reliably quantify the biological aging process will require incorporation of functioning/failure at various levels that can be integrated using systems level approaches. This talk will provide illustrations on how DNA methylation data (DNAm) can be integrated with cellular, physiological, proteomic, and clinical data to model age-related changes that propagate up the levels—finally manifesting as age-related disease or death. We will also show how network modeling can be used to generate a ‘diseasome’ model in order to identify hub methylation signatures with implication for multiple pathways and outcomes. Given the complexity of the biological aging process, modeling of systems dynamics over time will both lead to the development of better biomarkers of aging, and also inform our conceptualization of how alterations at the molecular level propagate up levels of organization to eventually influence morbidity and mortality risk.
Post-meeting Summary by Organizer[edit source]
Coming soon!
Daniel Promislow (Univ. Washington) Link to the source page[edit source]
April 9, 2019
Charge for working groups
- Come up with a list of major ideas/problems/concepts that you think we need to work on.
- Think about what conceptual areas could be linked to better address the major questions discussed in #1.
Apr 8, 2019
A few thoughts about general questions for discussion:
- What do we mean by "Biological Failure"? Aging? Senescence?
- Is there such a thing as a truly non-aging organism? An immortal organism?
- Things that change with age...
- Why do so many things appear to increase exponentially, and in parallel on a log-linear scale, with age?
- Are there commonalities across other levels of organization with respect to how things change with age, and by 'things', this could be function, or selection, or failure.
- What maintains variance among populations in aging. Even after controlling for G and E, we still see high levels of variance.
- Issues of complexity/simplicity and networks were touched upon today, but we have not yet gone into detail, discussing this.
Bernie Crespi
Suggestions idea that complexity can be bad. We can think of organismal organization (and function and failure?) in a two-dimensional space of couple (loose<-->tight) and complexity (linear<-->high). Berni suggests (I think) that biological entities with high complexity (brains, immune system) are more prone to bring the entire system down with failure. Entities that are simple and loosely coupled are less likely to fail badly. Suggests a negative correlation between senescence and intelligence, though the GWAS data supporting this are problematic (like the recent study claiming to find genes for SES--https://www.biorxiv.org/content/10.1101/457515v1) are likely due to social stratification. Bernie's neologism of the day: badaptation.
Dario Valenzano
Comparative analysis of annual life history in killifish. Would benefit from Nathan Clark approach to look at rates of protein evolution in annual/perennial species: https://elifesciences.org/articles/25884. Expansion of mitochondrial genome is striking. I wonder if the finding of increase in genome size in the annual species is true of annual plant species (like corn and rice, which have very large genomes) relative to perennial plants.
James deGregori
Notion of adaptive oncogenesis, with stem cells well adapted to niche. As tissue ages, stem cells are no longer well adapted to the niche in which they find themselves. I wonder whether these models are also relevant to the selection process that happens *within* a tumor once cancer growth (and mutator phenotypes) is underway.
Roz Anderson
CR animals show a few major clusters of correlated -omic features, while AL animals show a very large number of small molecules. We should discuss just what these correlations mean, both statistically and biologically, and why these correlation structures (adjacency matrices) differ among the two groups so dramatically.
Tulja
Finishes talk with three general questions:
a. How strong is the trade-off between added longevity and lost fertility
b. Can we explain environmental plasticity?
c. Does stochasticity matter? On any time scale?
For all three of these questions, I wonder if high-dimensional assays (metabolome, epigenome, etc) might have something to add to this discussion...
Sabrina Spencer
The technology that Sabrina is development could add tremendous power to the work now ongoing to track yeast cells as they age in real time. Also shows that quiescent cells are resistant to various stressors. Is that simply that they are metabolically quiescent and so not taking in these toxins?
Barb Natterson-Horowitz
Suggests that the disease-associated heart responses that we see could be maladaptive responses that evolved for adaptive reasons ('capture myopathy', 'alarm bradycardia'). Barb finds evidence for these phenomena in non-human species, but these could well be a large underestimate, simply because the vast majority of these events are never observed, and when observed, not reported.
Michael Hochberg (Univ. Montpellier/SFI) Link to the source page[edit source]
Rozalyn Anderson (Univ. Wisconsin) Link to the source page[edit source]
A theme that I see across the talks and in the discussion is the issue of complexity and how integrity of complex systems is lost with age and how it might be retained to impinge on health and resilience.
The idea of the adaptive landscape is very useful as is the idea of tipping point - i particularly like the idea of aging as a series of transitions where the path taken dictates the possibilities open for the future
Hochberg: Concepts that caught my attention, as a function of age is loss of resilience equally felt through the lifespan ie young v old? Also Diverse/idiosynchratic networks – how should models be informed. The idea of hierarchies of regulatory or adaptive nodes is interesting but I wonder do we know that there are grades of nodes in the first place, if there are how do we find them?
Crespi : Biological Risk Matrix… coupling versus complexity. I had difficulty with this idea because the systems were assigned importance but it wasn't clear to me what the basis for those assignations was. I have viewed the organ systems as different but inseparable pieces of the organism as a whole. I do like the idea of viewing the aging of specific processes in terms of trade-offs - I wonder about the inbuilt redundancy of systems and think we could consider the possibility that age-induced adaptions might just as well be beneficial - ie tailored to the prevailing internal environment or the current disposition of regulatory nodes.
Valenzano: The fact that ecology predicts genome size was super interesting and that the expansion is explained by transposons! I also loved the idea that the long-lived species had more emphasis on positive/purifying selection and the short-lived species had more evidence of the relaxed selection. These are an amazingly useful species for the interactions of genetics, environment - the exposome!
Di Gregorio: Among cancers age dependence in risk is shared despite differences in etiology and mechanisms of tumorigenesis and differences in the stem cell pools that these cancers arise from. All map to a common cancer curve – incidence as a function of age all lie right on top of each other. This nicely captures the idea that aging creates a ubiquitous risk increase for cancer incidence. Metastasis – moving from the environment where the tumorigenesis initiated – giant hurdle for success but likely to be huge number of cells that slough off. Idea that youth is associated with “Healthy Neighbors” proximal to the initiating cancer cells. Aging is not just accumulation of mutation: idea that the tissue changes create the promiscuous setting. Essentially: the behavior of a single mutation is not equivalent in young and old environments
Tuljapurkar: Response to challenge changes with age – makes the case that the amplitude of the response in young is muted and un-muted and over-amplified in aged, although I would think of it as a disconnection in the response whether that be under or over reactionary. I was very taken with the idea that response to a challenge could push you out of the equilibrium space and into a different state altogether & that you would need to consider the following: Depth of well; curvature of the well; size of the fluctuations.
Spencer: very interesting model for thinking about non-genetic sources of heterogeneity looking at individual cells through the lens of Proliferating v quiescence as a cell state. Spontaneous heterogeneity in asynchronously cycling cells used to identify key nodes in dictating the pace of cell cycle - really nice cell biology and time lapse imaging to uncover CDK2, p21, and stalled forks in the mechanisms. Interesting observation that Mothers pass damage on to the daughters so that the intent to enter quiescence already established in G2 of the mother. Genetic approaches to manipulate CDK2/p21 show that the slow cycling cells have higher stress tolerance – If you force CDK2 in the pausers have a fitness deficit - I wonder though if this is just because cells not ready for division were forced into it creating a vulnerability to stress rather than exposing a beneficial role for the pause.
Natterson-Horowitz: Using examples from different species the relative balance of sympathetic/parasympahtetic v vagal response to stress was explored. High-adrenergic events: eg sudden cardiac, death cardiomyopathy refelctive of a dysregulation of autonomic balanceTonic immobility in response to attach – seen in many different species. Alarm bradycardia is primarily a juvenile response. Not called syncope (because not people) but it sure looks like it. As the animals transition to adulthood they swap over to the sympathetic/parasympathetic response. I do wonder how this is coordinated and communicated with maturation - could there be a signal to indicate that a critical physical threshold had been reached for example, like myokines even? I like the idea that with age there may be a disconnect where there is a loss of the ability to toggle between sympathetic/parasympathetic and vagal responses☂Open questions are whether early experiences inform future balance in terms of response to trauma, Can we build predictive models?
OPEN DISCUSSION: Identified 3 themes: 1. Vulnerability with age, 2. Variance among individuals, 3. Complexity
Some of the ideas that caught my ear include the following: a) The theme of return to homeostasis; b)Changing landscapes with age – and changing landscapes with exposure, the Idea of tipping points applies here too, are there sets of perturbations that would allow you to track their responses and use that to predict the transitions among wells. Expectation that the well tracks with fitness may not be realistic – items roam around the ridges, c) idea that using “Layers” might be a useful way to parse the different components, can we use layers to frame the aging landscape – feedback loops among layers. This concept is likely important for understanding resilience, d) I really liked the analogy to collapse of ancient civilizations: ebbs and flows – tendency toward senescence – more complex, more overheads, more fragility. Is there a way to articulate loss of integrity or resilience and how it might be conceptually related to ecosystems or larger scale entities? so you might think of system failure as an emergent property, e) Nailing down the semantics – framing the language by context would be helpful for developing strategies to move these ideas forward.
Levine: Changes on the molecular level propagate up through the system. I really liked the concept that some degree of failure is tolerated up to a point. Important goal to provide an endophenotype that allows evaluation of intervention efficacy, provide insight into aging - the strategy is to work with risk identification – biomarkers of vulnerability. The methylation "clock" has been developed independently by several groups. Want it to predict more than chronological age so the goal now is to capture the true residual. There is some fascinating biology during development: under age 12y get a boost in methylation that has steeper trajectory from about 15y on the slope is consistent up to 60y. Interestingly, clocks with highest association to chronological age do not have the best predictor ability for disease when age is used as a covariate ☂. Looking now for gene networks – weighted gene correlation networks and then establish associations among networks and the various clocks – amazingly mitochondrial function OxPhos is a primary network ☂. Great next steps will be to take any given module and ask how will they are conserved across the clock – see congruent and discordant depending on what is being looked at for a given context- or in other words, which piece of the clock is driving the associations at different age group and diseases – building “disease-ome” maps.
Schneider: In traditional strategies to determine the resilience of hosts you infect and look at the dynamics of load and symptoms make a regression – generate a tolerance curve. Works at the population level but not at the individual level. To get around this employ a strategy where you generate phase plots – hysteretic curve- this nicely demonstrates that at each point in the infection you ae in a different space- curved around arrow. There was a wonderful theme centered on how to best visualize complex challenge and recovery data - so creative! One idea was to explore Resiliance/Tolerance/Robustness versus Frailty. Taking this a step further you can add new dimensions – generating a disease manifold. Superimposing genetic diversity on this framework revealed the beuatiful underlying biology: Cytokines, metabolome: orotate, parasitic density, granulocytes, reticulocytes. See basically the same changes among the different mouse strains but occurring at temporally distinct phases but always tracking with the course of infection and recovery. In a higher dimension see a wave move through the system – like ripples on a pond. As the shape of the wave changes that indicates where you are in disease course, if the order is perturbed then that might indicate morbidity. This is perfectly set up for exploring aging and comorbidity in the loss of implementation of the infection response and recovery. Some very interesting metabolism is at the heart of this response. Parasite load doesn’t correlate well with outcomes across lines but metabolic status going into the infection does.
Scheffer: Resilience: capacity to recover from challenge, or how quickly homeostasis is recovered after perturbation. Idea of brittleness. System state v conditions (and their interactions)– tipping points where you have two basins in unstable equilibrium – loose resilience when basin of attraction becomes small ☂. An interesting example is abundance of particular bacterial group in the gut, the landscape changes with age and there are apparently tipping elements in the human intestinal ecosystem. As you age more likely to have a particular population distribution. A really fascinating idea is that of Universal rules that govern critical points – true for all dynamic systems where critical slowing down occurs at mathematical bifurcations. The idea is that there may be generic early warning signals for tipping points that could potentially be used for determining indicators of resilience.Using this concept, you might investigate natural fluctuations – the uniformity and small scale of resilient systems becomes increased in variance in the less resilient and so the fluctuation change serves as a dynamic indicator of resilience. The emphasis is on patterns of micro-recovery that inform of the dynamics of the system rather than on the state of the system. If the system as a whole reduces you then get more cross-correlations between subsystems. Failure in one system is communicated to other connected systems. This is a really clever idea and a terrific match for understanding the slippage of system integrity with age.
Bernie Crespi (Simon Fraser Univ.) Link to the source page[edit source]
(1) General problem/question
DNA/RNA/proteins - cells - cell-to-cell interactions - tissues - organs - organ systems - whole organism - groups of related organisms as in colony - community including non-relatives
how/why does senescence occur at these different levels, in the contexts of antagonistic pleiotropy and mutation accumulation? does balance of early benefits vs later costs vary across these levels and systems?
does the complexity/stability/entropy of the system matter? how?
why does senescence accelerate exponentially in humans - synergisms between failure types/levels? why up so fast around age 60-65 in particular (later for women)-becoming less useful as a helper/contributor/transfer-er then, and are being 'replaced' these roles by next generation?
(2) Caloric restriction - reduces deleterious aging effects on health but does not lengthen lifespan if started after adulthood - why not? increases investment in maintenance, reduces growth and reproduction, based on energy, thus mitochondria
all cells use energy - does energy restriction impact some cells/systems more (eg brain if not fully protected); or are all systems just ramped down - how does this impact on disease risks, exactly - do diseases require more energy? or are fewer mistakes/mutations made?
(3) Single cell talk p21. Is this work key to cancer biology in that tumor suppressor functions being lost leads to all cells just keeping reproducing? can one show that senescent cells are less likely to transform, using this system?
(4) Idea that senescence is an emergent property (of defense against worse failures) appears quite important to me
(5) Comparing biological failure between organisms and higher 'levels' (societies, civilizations, ecosystems, etc): how might societies/civilizations age in same way lower-level systems do? maintain current system even when its benefits decline, due to environmental or other change? external causes of increased weakness of units at different levels? Fit with Jared Diamond's ideas about Collapse of civilizations? (what were those need to check).
Parallel causes, biology and higher levels - complexity and coupling of components? Too much investment in defense? (Russia 1991)
DAY 2 questions
how do CR genes relate to antagonistic pleiotropy?
what are roles of germline mutation in relation to somatic mutations in senescence?
intelligence/brain size related to aging/senescence both within and between species (and related to CpG calendar/clock methylation) - why? do immune systems have 'intelligence'? (measure of their efficiency of functioning in some manner).
why length of post-reproduction life vary (esp females) - fewer stresses when young? more resilient senescence system? other? related to intelligence (more than pre-menopause lifespan)?
age around 60 represent tipping point into accelration of aging effects/disease risks? synergistic failure across systems?
adaptive landscape model - flatten with age as selection weakens, lower adaptive peaks? mutation moves you downhill easier?
prioritization of organ systems with age - how vary - costs of degradation is each system? brain, immune at top.coindcides with ability to trade off across systems, higher->less prioritization
aging and loss of information in networks, higher entropy, protein folding, feedbacks in networks, how measure these changes, chaperones, perturb system like insulin challenge test in pregnancy
DAY 3 thoughts, questions
How well does theory interface with data in study of aging?
Agendas of proximate-mechanisms, biomarker geroscience researchers how much integrate evolutionary or other theory insights, how useful will they be for advancing theory?
Empirical ideas
Crispr on CR genes then analyze behavior of cells/tissues across age
CR genes for maintenance (rheostat) how much trade off with growth and reproduction genes, strong theory predictions here
[aside: do empiricists who focus on specific function tend to not think about tradeoffs (in evolutionary contexts) because they involve competing/beneficial but incompatible functions?]
DIfferent organ systems ought to senesce at different rates
James DeGregori (CU Denver) Link to the source page[edit source]
Overall thoughts: A system can take a certain # of hits. A lot of failures are linear with age on a log scale. Why? Resiliency? Due to redundancy? What explains variance in a population? Natural selection has acted to limit physiological decline to the point that maximizes reproductive success (balanced against costs). So risk of physiological failure are not zero during reproductive years, but are minimized, certainly relative to competing risks for most of our (or other animal's) evolutionary history.
Bernie Crespi presented on how selection works stronger on the weaker components. Slow, invisible weaknesses increase over time. Can engender exponentiality of decay. Or is it that damage and mutations accumulate over time? Risk of failure is related to the degree of complexity in a system, and tightness of coupling between components. For example, power grids are tightly coupled but not hugely complex. Aircraft are both coupled and complex. Mental disorders represent alternative attractors in a highly coupled system. For immune system, autoimmunity, chronic inflammation, cytokine storms represent alternative attractors potentiated by tight coupling. Both can have diseases associated with over-defense. Both do not trade off well with other systems. Immune system is under greater positive selection, relative to brain system which is more stabilizing. Thoughts: I would ask how the failure rate of systems, complex or not, is impacted by environmental disconnects. For example, failures in the immune system (like autoimmunity) may be common today, but were not so common in ancestral times (re. hygiene hypothesis).
Dario Valenzano described how relaxed selection shapes the rate of aging across species. Killifish get tons of cancer late in life; almost all die with cancer. Not clear if they die of cancer, just with cancer. Some species live in areas where water is available for a few months of the year. Have drought resistant embryos. Can desiccate embryos. Hatch when rains. 5-6 rounds of spawning. Wetter areas – longer lives. Did a lot of genetic mapping. QTL of longevity. Can do crosses and single alleles can have big effects. Show heart, skeletal, and many other aging phenotypes. See evolution of life spans multiple times across killifish species. So can filter out phylogenetic relatedness. Short lived (annual) species have a larger genome mostly due to expansion of transposable elements and introns. A lot of LINE expansion. Probably due to reduced purifying selection. Much more of coding sequence is under purifying selection in non-annuals. Populations evolving in dry environments have smaller Ne - even within the same species found in dry and wet environments. See expansion of mitochondrial genomes in annuals too, with a higher mutation rate (again consistent with weakened selection). In fact, the mitochondrial polymerase is more error prone. The question that I would raise is WHY is selection weakened? Clearly, a smaller Ne would do this, but why is there a smaller Ne? Is there a bottleneck with each season where only a small fraction of dried embryos survive and reproduce again?
Shripad Tuljapurkar – As we get older, get more response to similar challenges. My thoughts - but do we? Or do we simply return to homeostasis more poorly? In fact, sometimes responses are exaggerated and prolonged (such as with inflammation) in old animals. Escape from “well” for a phenotype is dependent on depth of well, size of fluctuations, and multidimensional space. Old age involves shallowing of healthy well and deepening of sick well. Thoughts: the well may also be broader, with a greater diversity of phenotypes (cellular) for the same cell type. Relaxed purifying selection, as well as epigenetic drift, could contribute to this.
Sabrina Spencer discussed how replication stress and DNA damages induces p21 in mother cell (somatic cells in culture), leading to increased p21-high offspring which stay quiescent longer. And these cells are stress resistant (survive better D dam). See much less pausing at early passages of foreskin fibroblasts relative to late. Thoughts: It will be interesting to see how these dynamics contribute to tissue aging. Also, in a competitive tissue do these same cells with DNA damage get purged? There is evidence from flies and mice that that damaged cells can be pushed out by healthy neighbors. From discussions during hike, it would be great to have a method to identify cells with critically short telomeres in a population (such as by marking gH2AX colocalized with a telomere binding protein - but not feasible for living cell...).
Summary of Day 1:
Themes: 1) Basins of attraction, 2) Failure (gradual, rapid, can we predict?), 3) Underperform or misperform, 4) Tradeoffs and pleiotropies, 5) Networks, not discrete compartments. 6) complexity vs simplicity; complexity vs complicatedness, 7) Don’t make a part that consistently outlives the whole.
Day 2:
Morgan Levine – DNA methylation landscapes in aging and disease. Changes on the molecular level propagate up through networks, increasing risk of disease. Every level above can tolerate some level of failure below. Biological aging proceeds, phenotypic aging, which proceeds functional aging. Biological age can be different from chronological. Use epigenetic clocks – methylated CpGs. Measured at 353 CpGs throughout genome. R=0.98 for chronological age. Do correction for <12 year olds to account for rapid change in early life. Developed “phenotypic age” predictor of aging related mortality based on clinical measures, which can predict disease. Had almost 10,000 individuals aged 20 and up, with up to 23 years of mortality follow up. Combine 9 clinical markers (albumin, CRP, MCV, etc). Incredibly predictive; 1 year increase in biological age coincides with a ~10% increase in risk of CVD, cancer, diabetes and lung disease. Comparing top 10% to bottom 10% of a group shows ~2x difference in HR. Most people will fall within 5 years of their chronological age. And difference remains stable. For all cause mortality, get about a 1.05 HR (per year of change in biological age, I think). Correlations with DNA methylation clock too. Horvath clock corrects for differences between tissues (there are differences). Developed transcriptomic signatures, correlated with DNAm clock. Cellular respiration, lipid oxidation, and nucleotide metabolism correlate well with biological clock. Thoughts: how do changes in DNA methylation correlate or not with changes in DNA mutation accumulation? Some data from Sanger indicate that the increases in mutations from birth to death are linear, but epigenetic changes show a sharp increase during growth/development periods. Why? How can we take these results and make them useful for the individual? It's not enough to just tell someone they're going to die earlier than their chronological age suggests, but what could be done about it?
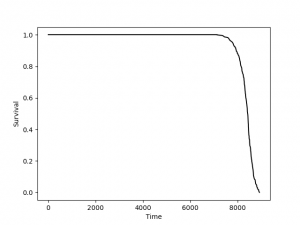
David Schneider – measuring resilience of hosts to disease. Can measure tolerance to an infection. Can we predict trajectories for individuals? Not yet, but can for populations. Looking at tolerance across 150 mouse strains from crossing 8 different inbred strains. If plot NK cells by RBC, get loop. Can figure out where someone is on a “disease clock”. Can see wave of parameters, as one rises while the previous falls. IFNg is first, gamma delta T-cells last. Kynurenine comes on when animals are sick. Orotate is late. Can look at curves for different mice strains (some die, some survive); 29 metabolites correlate with resilience (can predict who will die and who won’t). If arginine bottoms out, the mouse will die. And arginine really is critical for surviving infection. Kynurenine pathways is also critical (as it is for aging). Parasite load correlates poorly. If treat with anti-parasitics before d6, reverse on curve. But not if after. Also showed that glucose can prevent deaths from infections (provides energy). Our mouse houses are at 20 C, while prefer around 30 C. Get hypothermic upon infection instead of fever. Check out his blog: Phasecurveblog.wordpress.com
Thoughts: how do we alter responses so that recovery is favored over failure (death)? Can we manipulate metabolites? His and perhaps other labs seem to be doing this.
Marten Scheffer – ecology of resilience. How quickly is the return to normal after perturbation? He studies lakes. Sometimes they don’t recover. Due to severity of insult or the brittleness of system. Catastrophe theory on tipping points. How to know a stability landscape? For example, a tropical rainforest can be become less stable if there is less rain. If plot tree cover with rain across Earth, not a simple correlation, but discrete clumps – attractor states (dips in landscapes). See for bacteria in human gut with age. Basins of attraction are shallower in old age – smaller disturbances can push out to new state. Greater resilience can reduce cross talk between systems (negative cross effects). Can find early predictors of climate change, depression (mood), etc. Do we need to make perturbations in humans to predict future health? Thoughts: how do manipulate resilience? Can we increase it or at least prolong it later in life?
Wrap up: Develop frameworks for understanding biological failure. Can we derive concepts that inform models? Bernie – can connections be made between larger theories (Antagonistic Pleiotropy and Mutation Accumulation) with molecular changes like in epigenetics. What are patterns of change? Why do they change? Feedbacks maintain homeostasis and restore it post injury.
Hmmmm. Why is so much of what bad happens to us as we age exponential?
Our charge: Frame what we agree on, what are the big questions, and what we could do to get answers (with unlimited resources) and what can we not answer?
1) Try to come up with fundamental and important outstanding questions in term so biological failure?
2) What are the kinds of connections that we can make between conceptual approaches that will allow us to work on these?
Morgan Levine (Yale Univ.) Link to the source page[edit source]
- Considerations of time-scales. Where/how is time encoded?
- With time, new structures emerge, network connectivity changes
- Can hyperactivity that optimizes growth and development contribute to aging (antagonistic pleiotropy)
- Peto’s paradox—what things besides the number of cells, which should influence probably of neoplastic transformation, confer differences in species-level cancer risk?
- Only the intercept, not the slope for the age-specific rates of lung cancer differ between smokers and never smokers.
- May not be intrinsic damage accumulation and instead may be the difference of the “aged” vs. young environment which differentially selects for oncogenesis
- Clonal hematopoiesis could be facilitated/accelerated by the old versus young environment?
- How does this fit in to the findings from heterochronic parabiosis? Would changing the environment of the niche from old to you or vice versa alter 1) clonal hematopoiesis, or 2) probably of neoplastic transformation? What is the impact of HRAS in young versus old environment?
- Aging may be both the small perturbations to the system and the reaction to perturbations in neighboring systems for which one depends on. With a healthy neighbor idea, a small perturbation may not matter if the interacting system is functioning well, but ass the systems all go down hill together, there will be feedback that will cause an acceleration in the rate of multi-system declines. This may account for why things that change linearly with age, produce exponential morbidity/mortality cures at the level of the population.
- CR is changes a lot of pathways that together produce both lifespan and health span extension.
- One can start to map the CR response to systems-level (or network) changes involved in a variety of pathways.
- Interesting overlap with the genetic architecture of insulin resistance
- The same pathways that come up in CR are also the ones we are finding relate to the epigenetic clocks in humans. Also evidence that CR decreases mouse epigenetic age.
- Wells that define healthy vs. sick and transition out of a well depends on depth, curvature, and the size of the fluctuations.
- A hidden Markov is a good way to model this because the transition to a given well/state depends upon the current state—doesn’t matter how you got there just the parameters above (I would also add that there are likely many well and proximity to a well influences probability of transition).
- I would ask whether the overall landscape is changing, or whether the available landscape (what is in your vantage point given where you are) is the only thing that changes. If we think of the wells as discrete states, they shouldn’t actually change, just your probability of sampling a given space given your current space will change.
- Can you also model entropy using these wells? Basically you will have a tendency to move lower in a landscape towards equilibrium and moving uphill requires energy input.
- Does it make sense to consider single-cell data and investigate the probability distributions of having a multi-dimensional profile as a function of age (could use something like Mahalanobis distance).
Barbara Natterson-Horowitz (Harvard) Link to the source page[edit source]
Today's lectures spanned subcellular level to ecosystems. After today I find myself increasingly in exploring whether and how well conceiving of aging as a narrowing of the range of dynamic responses to environmental change works 'below' the organismic level.
Tomorrow we will define aging---it will be important to separate what are 'wear/tear' effects (calcification, fibrosis etc etc. etc.), what are from longevity issues.
Questions:
Is there underrecognition of the role of induced developmental pathways during pre-scenescent periods on characteristics of senescence
What is and is not sound about the hypothesis that for older animals with decreasing reproductive ability, engaging in activities which improve safety and resources of the environment (which would give their preexisting offspring advantage) might induce life-extending effects. Selection for longevity potential....amplified by induction of pathways by environment/community enhancing activity.
Marten Scheffer (Wageningen Univ.) Link to the source page[edit source]
The topic of aging turned out a really nice bridge between disciplines indeed. I thoroughly enjoyed learning about aging mechanisms that I had only distantly heard of and all that from such a wonderfully diverse group.
Reflecting on the relationship to the working group on 'multi systems human aging' within the arrow of time program and a workshop that I ran earlier with some of the same people (geriatricians, psychiatrists, animal scientists, critical care doctors) I was struck by the complementarity.
Our current group on biological failure talked mainly about the the mechanisms that affect the near-universal 'slow creep' that aging causes on a cellular level throughout the body. By contrast the other group was mainly interested in how the network of subsystems that regulate critical parameters such as mood, posture, blood pressure and temperature can lose resilience and collapse. It seems to me that those aspects might shed more light on the wide variation in health outcomes for otherwise similarly aged (on a cellular level) persons. It could be cool, in the coming years to convene a workshop that combines the interests of the two groups. It would stretch the diversity of viewpoints even further, but the common thread of aging should ensure that we get another exciting synergy going.
David Schneider (Stanford) Link to the source page[edit source]
Some ideas I had in response to talks and conversations:
Morgan said something about organisms continuing to age post death. There might be work to support this in Drosophila and demonstrate that it is under evolutionary pressure. Dan Hultmark made an argument that the fly's immune system wasn't good at fighting pathogens, rather it just prevented the fly from turning into compost before death as it defended against the microbes that decompose the dead fly.
Daniel mentioned "The secret lives of trees". Sometimes it it useful to look at fiction that explores these ideas to see what could happen if you don't worry about having to do the experiment. If that sounds interesting there are a couple of novels worth reading. Powers wrote "The overstory" that deals with plant interactions with other plants and humans in which the plants are the main characters. Likewise, "Semiosis" imagines humans colonizing a planet where the plants are far more intelligent than the humans and manipulate the people.
I finally got an explanation as to why I haven't been able to see critical transitions in my data. It looks like my trajectories are too dynamic and multidimensional for this to work, which is good to know because it was going to be very difficult to gather data and the necessary rate.
Someday I would like to see a method of showing how a network can evolve over time. I'm not sure of how to do except by showing a movie. It would help me understand how the connections in a network change with age.
I'm wondering about social interactions beyond loneliness that could affect aging. Are there social behaviors, that are the equivalent of monkeys grooming each other that humans perform that can increase resilience?
Dario Riccardo Valenzano (Max Planck) Link to the source page[edit source]
Day 1
DPromislow: Evolution shapes function and failure. Three dimensional space: Failure, Function and Evolution.
Main question: why do different agents age at different rates (faster, slower?)
MHochberg: Function criticality, aging and resilience. Coupling mechanisms of adaptation and aging. Wait, what are we referring to here for adaptation?
James DeGregori
Is it evolutionary Explain this! Oncogenic mutations in young healthy stem cell
populations typically reduce cellular fitness.
Peto's paradox: large and long-lived animals do not develop more
cancers.
Cancers requiring different numbers of driver mutations and
originating from vastly differently organized stem cell pools
demonstrate very similar age-dependent incidence.
Adaptive oncogenesis: we evolved stem cells that are well adapted to
the tissue niche. Stabilised selection for the evolved type. This is
more powerful than avoiding mutations. Stem cells would be adapting to
new environment.
q: does it mean that changing the tissue environment you would lead
stem cells (cancer) to evolved towards optimality in the "healthy"
environment?
q2: how about metastasis? do they evolve new-niche specific
variations? or physiological adaptations?
Rozalyne Anderson
Conserved pathways responsive to caloric restriction, across tissues.
Chronic low-level mitochondrial activation in cells. Mito regulator
PGC 1A (expressed to 1.5X, similarly to CR tissues). Super cool!
Tulja:
- Frailty and disability: transitions
- Post-reproduction lifespan
u(a, H) < u(a, S)
u: death rate
a: age
H: health
S: Sick
Recovery becomes harder as we age.
Decline in homeostasis with aging.
Potential well model (similar to fitness lanscapes)
Escape from potential well. Fluctuations depend on well depth, curvature of well and size of fluctuation. It's a multidimensional space.
Prob (H --> Ha)
Prob(S --> Sa)
As we age, the profile of the H and S well changes, making it harder for the ball to escape from the S well.
Longitudinal data on health, frailty, morbidity. HMM drive transition states. (Aging studies, Framingham, HRS, ...).
borrowing Hamilton's fitness (grandmother effect, old men, learning, transfers).
Sabrina Spencer
Causes and consequences of non-genetic heterogeneity.
How do cells switch back and forth between quiescence and
proliferation. Shift from G1 towards G0 depends on CDK2 (off). She develops awesome trackers for CDK2. Wow!
Bifurcation in CDK2 activity appears in many cell types.
p21 inhibits CDK2. p21 -\- remove the bifurcation, leading to proliferation
only, no quiescence. Many quiescent cells have DNA lesions. Mothers of
quiescent daughters have a longer cell cycle. Daughter quiescence is
decided in the mother's G2 phase. Mother cells pass DNA damage to
their offspring cells (i.e. do not retain the damage).
CDK2low state fortifies cell lineage against stress.
Barbara Natterson-Horowitz
Cardiovascular disease in humans, non-human primates, lions sympathetic and parasympathetic system.
Sudden cardiac death (SCD), "Tahatsubo" cardiomyopathy.
High adrenergic events. Sympathetic responses are flight and fight. Parasympathetic responses are opposite: faint, shit yourself, etc.
Fainting: vasovagal syncope (VVS), due to underprofusion of the brain.
VVS: paradoxical bradycardia has developmental characteristics: depends by the age of first fainting.
Alarm bradycardia is preferentially a juvenile phenotype.
Early life events: shaping autonomic nervous system for later events.
Day 2
- Intro by Michael Hochberg
- Basins of attraction - Failure, gradual, rapid, can we predict it - What is failure, underperformance or misperformance - Tradeoffs and pleiotropy - Networks - not discrete comparts - Complexity vs. simplicity - Henry Ford Aging and senescence, time and disfunction. SSpencer: Immortality, regeneration and rejuvenation. DPromislow: Michael Rose's concept of age-depedent decline in fitness components.
- Morgan Levine
Geroscience. Hallmarks of aging that accumulate over time. Systems biology of aging, changes ion the moleucular level propagate through the networks. Preservation of function (paper with Luigi Ferrucci in Circulation research). Aging heterogeneity, chronological age is an imperfect proxy of the latent concept, biological aging. Epigenetic clocks. Chronological age has been shown correspond with distinct chagnes in DNA metylation at specific CpG sites. Horvath's and Hannum's clocks. These clocks are done using supervised machine learning. She wants to capture the true residuals, rather than minimizing the residuals. Understand whether those residuals tell you anything about the underlying biology. Getting more biology than just chronological age from the clocks. Phenotypic age, predictor of aging-related mortality based on clinical measures. The used markers are albumin, creatinine, glucose, c-reactive proteins, lymphocyte percent, mean cell volume, red cell ditribution width, alkaline phosphatase, white blood cell count, age. Consensus co-methylation networks - data from 6 different tissues. 14 modules, showing age correlation for each CpG. Which piece of the clock matches what tissue, etc. Diseaseome map.
- David Schneider (Stanford)
Resilience and disease space. How sick are you going to get with a given load of pathogens? Symptoms = f(microbe load). It's a disease tolerance curve. However, tolerance is a population measure and does not apply to individuals. How do we expect disease dynamics to vary? All models are wrong, but some models are useful (George Box). [[1][Tracking Resilience to Infection by Mapping Disease Space]]. Using a range of mouse diversity to identify imporant regulatory mechanisms. 8 parental mice used to generatel RIL. He brings up that methylation clocks are actually calendars, not clocks.
- Marten Scheffer
How ecologists have been looking at resilience. Resilience: the capacity to get your acts together, to recover after damage. How quickly things come back to normal after perturbation. Sometimes things do not recover from perturbation and does not come back. Another way to think about it is that the system has become brittle. Unstable equilibrium. Theory developed in the 1960s, catastrophe theory. Salvador Dali and his own tipping point. Holling in 1973 thought about resilience. How to know a stability landscape. Tipping elements in the human intestinal ecosystem. Generic early warning signals = indicators of resilience. Critical slowing down. Dinamic indicators of resilience (DIOR). A bird shits on your head. After a hour you're ok, it means you're resilient. If after a hour you still hungry, you're not resilient, you may be depressed. This is based on patterns of micro-recovery. Fast recovery, slow recovery. Temporal autocorrelation help you predict tipping points. The previous arrow of time group has looked more at the complexity. This group is more looking at the molecular side.
- DPromislow
Common dynamical phenomena underlying description of the causes of failure. What are those communalities? This can help us get to the causes and dynamics of failure. This could be an argument of discussion. Group discussions to address communalities and differences in failure.
- Group Discussion 1
Can we reverse, halt, or slow down aging or is aging inevitable
- which components? Epidermis.
- ways to slow aging: CR
Is aging inevitable? Entropy. Does entropy at individual level
increase, causing aging? Individuals are open systems.
What are the components of aging?
How do we distinguish between intrinsic and extrinsic causes of aging?
Intrinsic: failure in DNA repair, proteostasis.
Extrinsic: falling down, UV light
Do you really return to the same initial state after a perturbation,
or are you missing a dimension.
The landscape that David Schneider showed may change as aging.
progresses. Contours over which these things occur may be morphing.
Day 3
Sabrina Spencer (CU Boulder) Link to the source page[edit source]
Day 1.
Bernie Crespi.
Accidents (failure) are inevitable (normal). Challenger disaster, Max 8 jet. Risk of failure depends on complexity of the system. Tight coupling vs modular. Tight coupling – butterfly flaps wings causes a storm elsewhere.
Plot of tightness of coupling vs complexity. Brain and immune system go in top right corner. Is biological failure mediated predominantly by the brain and immune system?
Can make this plot at top right corner at the cellular level too.
SLS: how to test resilience of cells? Can we come up with a set of perturbations and appropriate responses? A stress test for humans before a surgery, or for populations of single cells?
Disease and senescence anti correlate with intelligence. Correlation between intelligence and lifespan is mostly genetic. A result of having more ‘good’ genes. Peter Visscher lab 2016.
Mental disorders represent alternative attractors due to tight coupling. Schizo, depression, autism.
Neurons have to be well-defended bc they are long lived and aren’t replaced.
Neuronal stress: age, apoe4, infection, energetics, insulin resistance, sleep deprivation.
Bodily senescence is mediated by over-defense and inflammation.
Failure due to bad tradeoffs.
Is senescence due mainly to defense against death? The defense ends up killing you.
The immune system is what is keeping people alive in the face of infectious disease.
Loss of coordination among organ systems causes health risk. They are networks. brain is made up of many different kinds of cells including immune cells.
Dario Valenzano.
Relaxed selection shapes the rate of aging across species. How can we reverse time and intervene in aging?
Killifish live 4 months.
Some strains live 15 weeks, others 30 weeks. Cross them and get genetic maps controlling lifespan.
James Degregori.
Why do we get more cancer late in life? Not the accumulation of oncogenic mutations. Loss of tumor suppressors actually reduces renewal of stem cells. but past authors focused on increase in cancer.
Investment in tissue maintenance during youth.
New model of multi stage carcinogenesis where a mutation has a negative impact early in life and a benefit later in life. Bc a stem cell is not well adapted to an old lung and thus will evolve. The same mutation can be maladaptive early in life but adaptive late in life.
Bad cells are poorly adapted to their environment and are pushed out.
It’s not the cell, it’s the mismatch between cell and environment. Adaptation is more likely bc the system is not working.
Senescent cells are a huge part of the environment.
Adding another dimension to somatic evolution.
IL37 transgene is anti-inflammatory. *
Inducing a genetic translocation with crispr in young mice did not cause tumors. But it did if you induce in old mice. But not if you suppress the immune system.
Quality control goes down late in life.
Lawrence Loeb showed that increasing mutation frequency by mutating DNA Pol delta’s proofreading function does not result in increased cancer.
We are loaded with oncogenic mutations. We are not avoiding cancer by avoiding mutations.
Can capture the effects of caloric restriction by increasing autophagy.
Rozalyn Anderson.
Caloric restriction (in the absence of malnutrition).
RNA processing is different in the caloric restriction group.
Push pull of metabolism vs growth… gets disconnected with age.
Increase expression of PGC1alpha in mitochondria by 1.5x to mimic caloric restriction.
Wound healing is slower in CR animals. They would not be vigorous in the wild. They do things more slowly. The animals are smaller so there’s a growth effect of CR.
Constant vs periodic caloric restriction? Intermittent fasting. Fasting and resilience research is growing.
SLS: How well can you capture the benefits of continuous caloric restriction by doing 12hr fasting?
The periodic ketogenic period is important. Can now look for panels of molecules and patterns of change.
Shripad Tuljapurkar.
Back and forth between evolutionary timescales and one person’s lifetime.
Assume we are the same today as the Romans were.
When we are young we can handle challenges (like missing the bus). We have a muted response to challenges when we are young. Older people worry about things and have higher amplitude responses.
People get less homeostatic as they age.
Recovery is harder as you get older.
Menopause has to do with the number of reproductive follicles that you are born with. (?)
Grandmothers; learning and wisdom. Theory of transfers. Can transfer care, knowledge from old to young.
How much added longevity can we explain from transfers? 15 years. 50yrs to 65yrs old.
Questions I got from the audience after my talk:
· Is high-p21 CDK2-low state a trap? Bc p21 goes up and up, suppresses CDK2 more and more, which makes it harder and harder for a cell to escape and re-enter the cell cycle.
· Would every cell go through the CDK2low state at some point? Can you develop a sensor that would turn on once a cell goes into that state once. (sc)RNA-seq to identify features of CDK2low cells that would be long-lived or permanently on.
· Does % CDK2low cells increase as cells become senescent? Use primary cells at increasing passages.
· Have you normalized p21 and 53bp1 curves by CDK2inc? Set CDK2inc trace to 0 and look at points of convergence with the other 3 subpopulations.
· Similarity to yeast aging and asymmetry of mothers-daughter division?
Barbara Natterson Horowitz.
High adrenergic cardiovascular events = stressful events.
Vasovagal syncope = Fainting. An external event (like getting blood drawn) triggers slowing of heart, vasaodilation, underperfusion of the brain.
Alarm bradycardia. Loss of tone and fainting is a life-saving event when animals are being hunted. Playing dead. It is primarily in juveniles.
In adults, stressful events lead to fight or flight, not fainting.
A developmental response. Until you’re able to run away successfully, there’s a parasympathetic response. 13 years old is age of highest fainting
High adrenergic events cause heart attacks. Spike during Northridge earthquake.
Heart rate variability.
Restore dynamic properties of autonomic nervous system with exercise or mindfulness meditation.
Trapped shorebirds – 10% have heart attacks (and die?) after being trapped with a net. Some species fare worse than others – can you use the differences to predict which patients will have heart failure at the next stress.
General discussion.
Themes that have come up:
Variance.
Potential wells. Hysteresis. A to B is not B to A. light stress vs strong stress.
Resilience / homeostasis.
Can you see how frail a person is before a surgery? Don’t protect the elderly from all stress, give them some slight challenges and some exercise.
Measures of potential wells. Cell-to-cell variability. Maintenance of a proper phenotype.
How can we compute/quantify potential wells?
Define health: Physiological, psychological, social.
What is surviving? The ecosystem.
Scales: Genes, molecules, cells, tissues, individuals, populations, ecosystems, civilizations
Why do cars age and collapse?
Tipping points.
What is aging? Relationship to immortality?
Day 2.
20190409 Day 2.
Themes that came up again and again:
1. Basins of attraction. Wide, narrow, deep, shallow. For sick and healthy.
2. Failure. Gradual, rapid, can we predict?
3. What is failure? Underperform, misperform
4. Tradeoffs. Investment in critical functions could be associated with aging.
5. Everything is connected as a network (eg organs)
6. Complexity (many knots) (vs complicated – many folds)
Let’s define aging:
Programmed development. As well as non-programmed stochastic decay.
Process of aging vs pathology of aging. Separate disease-related aging.
Chronological age vs biological age
How does body protect itself and make itself new again?
It’s an open system so you just age.
Lack of rejuvenation and regeneration.
But a 40 year old woman can make eggs that produce a 0 year old baby.
Aging is entropy
Aging vs longevity
A series of repair mechanisms for wear and tear.
Cataracts and fibrosis are pathologies of aging. These are repair mechanisms that allow longevity.
Aging is suboptimal adaptation to environmental stressors
Aging is an active response, not passive.
Don’t forget fitness.
Morgan Levine.
Geroscience. Failure starts at molecular level, each level up can tolerate some issues.
People age at different rates. Can we quantify biological age.
Epigenetic clocks. CpG methylation. Usually turns down transcription if it’s in the promoter.
Horvath clock. 53 different tissues. Many post-mortem. Measure 353 CpGs across the genome and get a strong age predictor.
Hannum clock was trained on whole blood.
Uses machine learning to minimize the residual of the fit to a line. Want some residuals since an r=1 gives no information (you wouldn’t be predicting their age, it would just be their age).
It’s exponentially changing under 15 yrs old, then linear.
Get phenotypic age, then try to infer biological age.
10k people. Take out accidental death and HIV.
Q: Why focus on all-cause mortality? And not likelihood of a specific disease? Bc age increases your risk of all diseases.
We have multiple biological ages across different tissues.
Most people fall within 5 years of their biological age.
People are stable. Age at one time point is predictive of age 9 years later. People age 1 year per year on average. People don’t reverse.
Levine clock. Can predict age at menopause.
Most of the CpGs do not overlap across the 3 clocks.
Want to cluster the CpGs into module to figure out what they represent.
SLS: do you do any longitudinal studies? Do take your own tissues over time and predict age?
SLS: have you compared these clocks to telomere lengths?
David Schneider
Measuring the resilience of hosts to infections by mapping disease space.
How sick will you get with a given load of pathogen?
Tolerance to microbes is a population-level thing. Can’t do with one individual.
Health is a stable state, death is a stable state, but sickness is a set of many transient states.
Resilience: 1. How stretchy the system is. 2. How far you can go before you break.
With age, the phase diagram might change such that a state that was survivable when you are young is no longer survivable when you are old.
Measure cytokines, parasite density, NK cells, RBCs, etc over the course of the infection.
Could potentially predict where in the trajectory a kid is who comes to the clinic with malaria.
IFNG is first, gamma-delta-Tcells are last.
29 metabolites before infection predict which mice will survive.
Looking for critical transitions.
Q: Do animals have to always go all the way around the curve? No, if you treat them with drugs before day 6, the curve reverses, but not after.
Can rescue ketogenic mice from death by providing glucose.
Now want to do it all in humans.
Q: have you done this in old vs young mice? Yes, in 70 week-old mice
Marten Scheffer.
Resilience. How quickly things go back to normal.
In ecosystems, sometimes they don’t recover from a perturbation. They become brittle.
Landscapes are a useful metaphor.
Tipping points – 2 alternative basins of attraction.
How do you know where you are in a landscape?
Look over every square kilometer at tree cover vs amt of rain. Scheffer Science 2011.
Forest is stable, savannah is stable.
Abundance of a particular gut flora increases with age.
Systemic resilience of humans and other animals. Scheffer PNAS 2018.
Can we infer fragility? Climate collapse, societal collapse?
Generic early warning signals = indicators of resilience
Critical slowing down happens at mathematical bifurcations. If you push a ball and the slope is shallow, it takes a long time to return to normal. Fluctuations are longer, are more correlated over time.
Resilience in mood if a bird shits on your head. One hour later, are you happy again or still down?
All systems, human, earth, are continually being perturbed.
SLS: Can we make use of low-level fluctuations in single cells and compute autocorrelation to calculate resilience? Can we use this to measure cellular age? **
Measure resilience by measuring small fluctuations over time. Systems close to tipping point show:
1. Larger fluctuations
2. Stronger autocorrelation
What the other working group talked about: Measure balance on a balance plate (elderly do worse); Measure blood pressure during rapid standing from sitting (blood pressure slower to return to normal in elderly). Once you are frail, it’s already too late.
How can you predict from autocorrelation how far you are from tipping point? You cannot measure the distance to the tipping point. Bc it depends on chance.
Kelley Harris (Univ. Washington) Link to the source page[edit source]
Meeting participants have had some productive disagreement about what exactly defines aging. Is it the entirety of the change that occurs between birth and death or perhaps beyond, or just a subset of the changes that occur during one's life? Two main categories of change occur during life: one category is a sequence of programmed developmental milestones including embryogenesis, puberty and menopause. In semelparous species, even death can be viewed as a programmed developmental milestone. Another category of change is deleterious degradation of function, manifesting as cancer, heart disease, weakening of physical strength and life-sustaining activity such as predation, and even increased susceptibility to infectious disease. Some, including Roz, argue that only the second category of change should really be called aging. However, it can be hard to prove that any type of age-related decline is truly random rather than programmed.
In the classical view of aging as the breaking down of the body, participants make use of analogies involving the breakdown of man-made machines, e.g. the failure of a one-horse shay or Henry Ford Model T. The one-horse shay is a machine rooted in folklore that is perfectly efficient because all components fail at once. To the extent that human bodies fail to disintegrate at once like the one-horse shay, are we maladaptively wasting resources on our slower-to-fail organs? Or is longevity more of a neutral side effect of evolving bodies that are robust to the challenges we may encounter during our reproductive lifespans?
Barbara's work challenges the mechanical breakdown view of aging by comparing physiology between species and showing that age-related "degradation" can sometimes be an adaptive response to a stress that can in theory occur at any age. For example, age-related thickening of the heart ventricle is a rampant cause of human death today, but it is physiologically rooted in a type of phenotypic plasticity that can help a young animal adapt to high blood pressure and still live to reproduce. This suggests that when we die of old age, we are dying of the most severe negatively pleiotropic side effects that inevitably accompany adaptations that outweigh the cost of dying in middle or old age.
James challenges the view of aging as random mechanical breakdown in a different way than Barbara does. He mainly focuses on the cancer mode of death and the random accumulation of cancer driver mutations as a particular mechanism of breakdown. In the classical breakdown view of cancer, oncogenes are essentially ticking time bombs that have a constant probability of mutating each time a cell divides. This implies that everyone will eventually get cancer if they live long enough, with an exponentially distribution of ages at incidence. However, James notes that there is no appreciable difference between 20-year-olds and 30-year-olds in their probability of dying of cancer, whereas in the exponential mutation accumulation model, the difference between these age groups should be comparable to the very significant difference between 60- and 70-year-olds. To explain this violation of the simple exponential health decay model, James proposes that a breakdown in the cellular environment occurs during middle age that allows precancerous cells to proliferate in a way the same cells cannot do in younger tissues.
Morgan's work on the epigenetic clock outlines one way in which aged tissues are different from young tissues en masse. She has identified a set of CpG sites that are differentially methylated between young and old individuals and whose methylation state predicts mortality slightly better than calendar age does. From her presentation, I couldn't tell whether young individual had less variation in methylation status than old individuals at these sites. In other words, does aging cause decay from a deterministic methylation state toward a random state, or does it look more like a programmed transition from one state to another? To the extent that methylation is decaying toward a random state, mechanical breakdown seems like a better analogy, but if the aged state is as low-variance as the young state, programmed developmental transition seems closer to what's going on.
Maria Riolo (SFI) Link to the source page[edit source]
Interesting conceptual idea organisms traversing a state space with multiple local attractors and one absorbing state (death), and aging as changing that landscape and thus the probability of transitions between states. Even if we just looked at aging as a plain old flattening of the landscape (or accumulation of noise in the landscape/transition probs?), that would already pop out properties like breakdown of homeostasis/loss of resilience to perturbations (previously attracting basins aren't as steep) and a propensity to reach regimes that were previously hard to get to (e.g. cancers). At first glance it seems like flattening the landscape would lead to more variability across the board, more wide excursions to various states, but maybe that's not quite right - I could also picture a scenario where loss of local variability -> landscape dominated by broad features that haven't eroded away -> loss of diversity/flexibility, effectively being left with a small set of wide highways instead of a larger set of little paths.
Related idea came up today: how do "near flat until sudden acceleration of risk" disease incidence v age curves arise from more gradually creeping molecular aging? Possible mechanism could be that idea of gradual landscape change leading to a threshold where falling out of the basin of attraction becomes much more likely. I tried a toy model over lunch: stochastic logistic growth process with gradually declining carrying capacity. What do survival times look like? Turns out they do get that nice elbow property - could imaging evolving how much you invest in repairs to slow the gradual decline tuning that elbow to an appropriate age of "I probably already died by other causes and my expected # of future offspring is low."
Ophelie Ronce (UBC) Link to the source page[edit source]
Bernie Crespi:
The hypothesis/framework presented that risk of failure increases with linearity and tightness of coupling in the functioning of a system is interesting and stimulating, but I wondered about how to quantify these two axes in a practical manner in order to test this idea. How easy is it to classify biological systems on these axes? How much of this classification is artificial? I was also puzzled about how to separate these processes (brain functioning, immune response, and others) given their interactions and integration; but if all these different systems are integrated, then does it make sense to attribute risk of failure to properties of each system? Can this approach be adapted to take into account this integration?
How to define complexity in this context?
The fact that some aspects of senescence may be due to overdefense in later age, seems in line with other observations and theories that senescence may be the result of protective systems dysfunctioning with age; I wondered about the role of our interaction with infectious diseases in shaping our biology and therefore our life history through these mechanisms.
I would like to learn more about how mental disorders match "attractors" potentiated by tight coupling; should read more about this.
Dario Valenzano:
A very nice study system in killifish populations and species where climate/aridity/habitat (temporal ponds) correlates with divergence in life history. Lots of different approaches (QTL, genomics, comparative analysis, phenotypic studies of aging, mutation rates estimates): overall suggest that populations/species living in drier/more ephemeral habitats have accumulated a high genetic load affecting aging and lifespan through relaxed selection, which may be due in part to their demography with frequent bottlenecks. Nice to see such a complete study showcasing how mutation accumulation affects aging evolution. Reminds me of the work by the group of Christoph Haag on daphnia (also in ephemeral ponds)); see reference attached.
James DeGregori:
claims for a change in paradigm in our way of understanding and modelling cancer: number of mutatations would not be so relevant, because of selection on malignant cells. Model of fitness landscape with different peaks, changing with age. What are the mechanisms that make malignant cells unfit in young individuals (niche? policing by other cells? Can we model the interactions between cells to generate such fitness landscapes and their change with age?
What does explain that the increase in rate of cancer on a log scale is linear with time, with the same slope: can we predict that? what does it require in terms of assumptions?
Rozalyn Anderson:
Effect of caloric restriction on lifespan in 40 years long term experiments in monkeys; growth and immune function intertwined. What is the effect of caloric restriction with infectious disease? More generally would a better integration of infectious disease-driven evoluton and life history/aging evolution be necessary?
Shripad Tuljapurkar:
Hidden Markov models driving transition rates could be fitted using longitudinal data on health, frailty and morbidity: could we then estimate how fitness or potential wells change with age?
Barbara Natterson-Horovitz:
Fainting in young individuals broadly distributed taxonomically; could be an adaptation to escape predators in inidviduals that have no other options; links with capture myopathy
What are the life history correlates associated with capture myopathy vulnerability; points to insights in considering relationships with other species (here predation) in shaping evolutionarily features associated with various conditions
Morgan Levine:
aging dynamics at different levels of organization or different aspects of aging (biological, phenotypic, functional) may be different; what are the articulations between these different levels and aspects of aging?
Idea that markers of biological age may be improve our predictive power of condition beyond predictions based on chronoogical age; changes in methylation with age; predictor of chronological age based on methylation age (R=0.98 idea that residuals may inform on condition, but not much residuals variation to play with? not much extra information? methylation not as predictive as biological markers; methylation increases in all tissues but not at the same rate with age: why and how is it related to function?
Schneider & Sheffer: in both presentations, slow recovery signals approaching a critical tipping point; can we get an idea about how close we are from the tipping point? Martin says that there are no obvious way. With systems that have no fixed points as equilibrium but naturally cycle, it is also harder to find signals of a critical transition
general discussion: questions that I like= 1) how to connect ideas about tipping-points/resilience/netowrks to classic evolutionary models of aging, 2) can we design simple models predicting exponential increase in rate of mortality with age? with constant slopes? 3) can fitness landscapes/potentail wells be more than useful images to help us think about aging? Can we parameterize these models? Can they be used to make more quantitative predictions?
other ideas: can we build models with both antagonistic pleiotropy and mutation accumulation and study their interactions? are there distinct predictions about collapses of networks based on these different source of genetic variation affecting aging?
role of social interactions at different levels in biological failure (cell to individual), role of interactions with other species (parasites, predatiors, mutualists)
Reference Materials by Presenting Attendees[edit source]
Marten Scheffer (Wageningen Univ.)[edit source]
Quantifying resilience of humans and other animals in PNAS 2018 is a review that covers ideas generated by a related workshop of animal scientists and medical researchers, focusing more on the level of networks of functions at the organ and organism level.
| Title | Author name | Source name | Year | Citation count From Scopus. Refreshed every 5 days. | Page views | Related file |
|---|---|---|---|---|---|---|
| Doi.org/10.1073/pnas.1810630115 | 0 | 1 |
Shripad Tuljapurkar (Stanford)[edit source]
1) Haworth et al show that heritability of a well-defined measure of cognition (hence related to the vaguer concept of IQ) changes with age. Such studies are more reliable than GWAS modeling.
2) Steiner & me show that there is lot of non-genetic heterogeneity in complex life cycles, and how to compute it
3) Steinsaltz & Evans show that stochastic"reliability" models of complex systems do NOT lead to particular "generic" patterns of failure. E.g., we don't get Gompertz from reliability models. Humans are not cars!
4) Etges et al show that genes act as clustered networks that change with age -- see
Etges, W. J., Trotter, M. V., de Oliveira, C. C., Rajpurohit, S., Gibbs, A. G., and Tuljapurkar, S. (2015). Deciphering life history transcriptomes in different environments. Molecular ecology, 24(1):151–179.
| Title | Author name | Source name | Year | Citation count From Scopus. Refreshed every 5 days. | Page views | Related file |
|---|---|---|---|---|---|---|
| Neutral theory for life histories | 0 | 7 | ||||
| The heritability of general cognitive ability increases linearly from childhood to young adulthood | Molecular Psychiatry | 2010 | 0 | 6 | ||
| Markov mortality models: Implications of quasistationarity and varying initial distributions | Theoretical Population Biology | 2004 | 0 | 1 |
Dario Riccardo Valenzano (Max Planck)[edit source]
| Title | Author name | Source name | Year | Citation count From Scopus. Refreshed every 5 days. | Page views | Related file |
|---|---|---|---|---|---|---|
| Demography of dietary restriction and death in Drosophila | Science | 2003 | 0 | 1 | ||
| In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming Cellular reprogramming by transient expression of Yamanaka factors ameliorates age-associated symptoms, prolongs lifespan in progeroid mice, and improves tissue homeostasis in older | Cell | 2016 | 0 | 8 |
Sabrina Spencer (CU Boulder)[edit source]
- Baker et al. Nature, 2011: Paper from Jan van Deursen's lab on delaying (reversing?) aging by clearing senescent cells in a mouse.
- Zhang et al. Cell Syst., 2016: 3 different models for aging in c elegans with evidence for 2 of the models, from Zach Pincus's lab
| Title | Author name | Source name | Year | Citation count From Scopus. Refreshed every 5 days. | Page views | Related file |
|---|---|---|---|---|---|---|
| Extended Twilight among Isogenic C. elegans Causes a Disproportionate Scaling between Lifespan and Health | Cell Systems | 2016 | 0 | 2 | ||
| Ageing-associated disorders | Nature | 2011 | 0 | 5 |
Ophelie Ronce (UBC) Link to the source page[edit source]
1) Tenaillon (2014) reviews the insights brought upon by Fisher Geometric model in evolutionary genetics: could it be useful as well for our understanding of aging?
2) Martin (2014) shows how and when Fisher geometric model of adaptation emerges from complex networks of interacting modules
3) Promislow and Moorad (2088) did use that framework to model aging; could it be used to address different questions about aging, dfferent attractors, resilience?
4) tipping points on one side and evolutonary theories of aging on the other have been discussed as distinct frameworks, which should be better connected; how tipping points may be affected by evolution was discussed in a recent review (interested in ecological tipping points mostly); it could be a good starting point to read Dakos et al. (2019)
5) another example of mutation accumulation affecting aging in daphnia in Lohr et al. (2014)
6) still another one on fitness landscapes but combined with data on antibiotic resistance evolution to address questions about how these fitness landscapes change with the environment/stress: check Harmand et al. (2017)
| Title | Author name | Source name | Year | Citation count From Scopus. Refreshed every 5 days. | Page views | Related file |
|---|---|---|---|---|---|---|
| The Utility of Fisher's Geometric Model in Evolutionary Genetics Phenotypic complexity: the number of statistically independent phenotypic traits an organism exposes to natural selection in a given environment | Annu. Rev. Ecol. Evol. Syst | 2014 | 0 | 0 | ||
| Fisher's geometrical model emerges as a property of complex integrated phenotypic networks | Genetics | 2014 | 0 | 0 | ||
| A theory of age-dependent mutation and senescence | Genetics | 2008 | 0 | 4 | ||
| Reduced lifespan and increased ageing driven by genetic drift in small populations | Evolution | 2014 | 0 | 1 | ||
| Ecosystem tipping points in an evolving world | Nature Ecology and Evolution | 2019 | 0 | 2 | ||
| Fisher's geometrical model and the mutational patterns of antibiotic resistance across dose gradients | Evolution | 2017 | 0 | 1 |